Progress Update: Reimagining the Science Behind
Mechanism-Based Target Selection & Small Molecule Drug Design
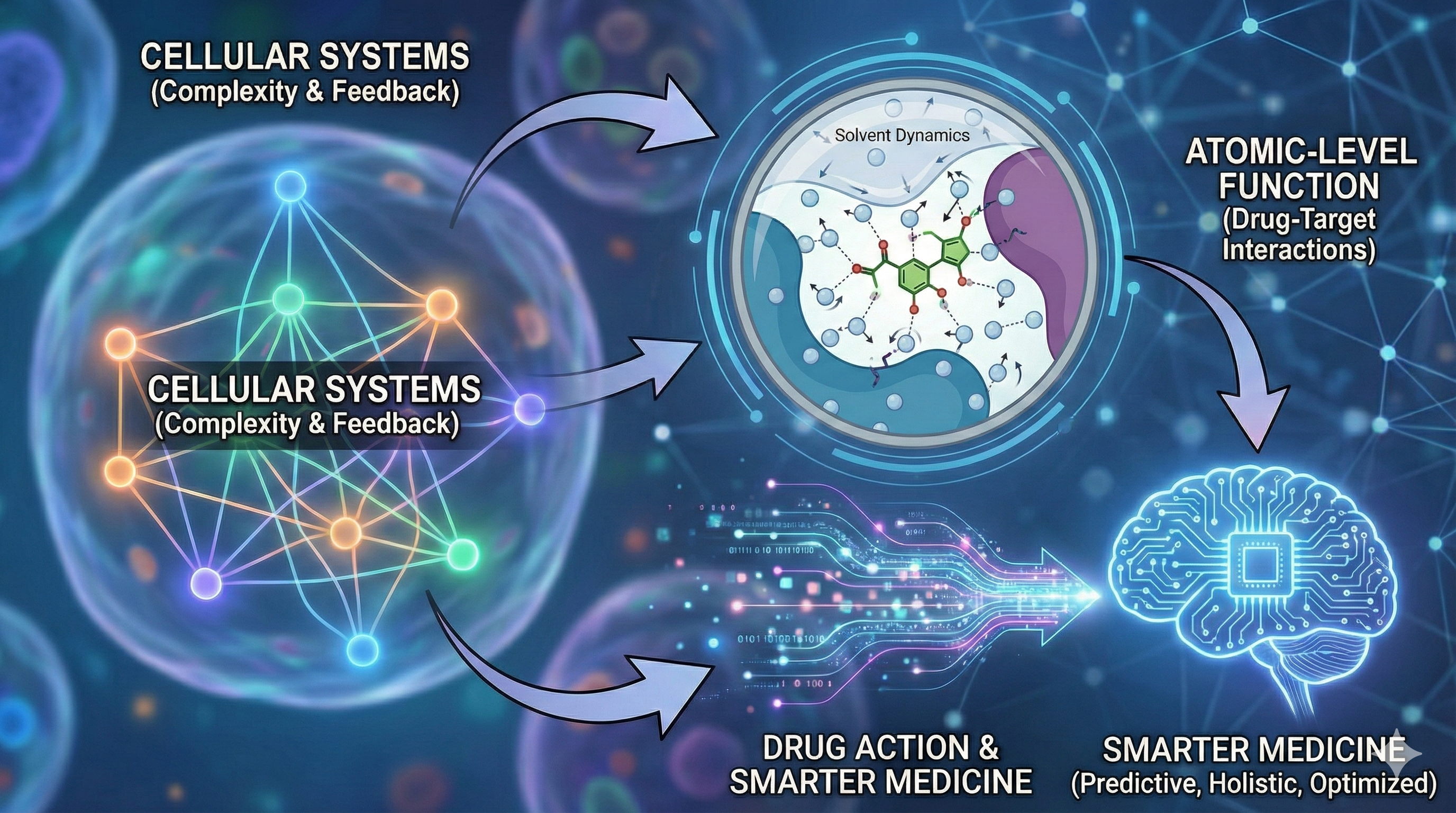
A new physics-based, holistic view: bridging cellular systems, atomic-level function, and drug action for smarter medicine.
Background: For over five years, our group has championed a holistic, first-principles, physics-based framework for understanding and predicting the complex molecular logic of cell (dys)function and its relationship to drug action—across both atomic and systems scales. This approach challenges the current status quo, directly tackles failure points in drug discovery, and drives new paradigms in both target selection and compound design.
What Sets Our Theory Apart?
- Top-Down/Bottom-Up Integration: We merge atomic detail (binding, solvation, molecular physics) with cellular network context, recognizing their mutual influence in living systems.
- Real-World Relevance: Focus on drug-target and drug-off-target binding under native physiological conditions—bridging a critical knowledge gap that hinders translation and safety prediction.
- Impactful Science: Our hypotheses and mathematical models, detailed across multiple publications and preprints, shine light on root causes of preclinical and clinical failures, going beyond incremental improvements.
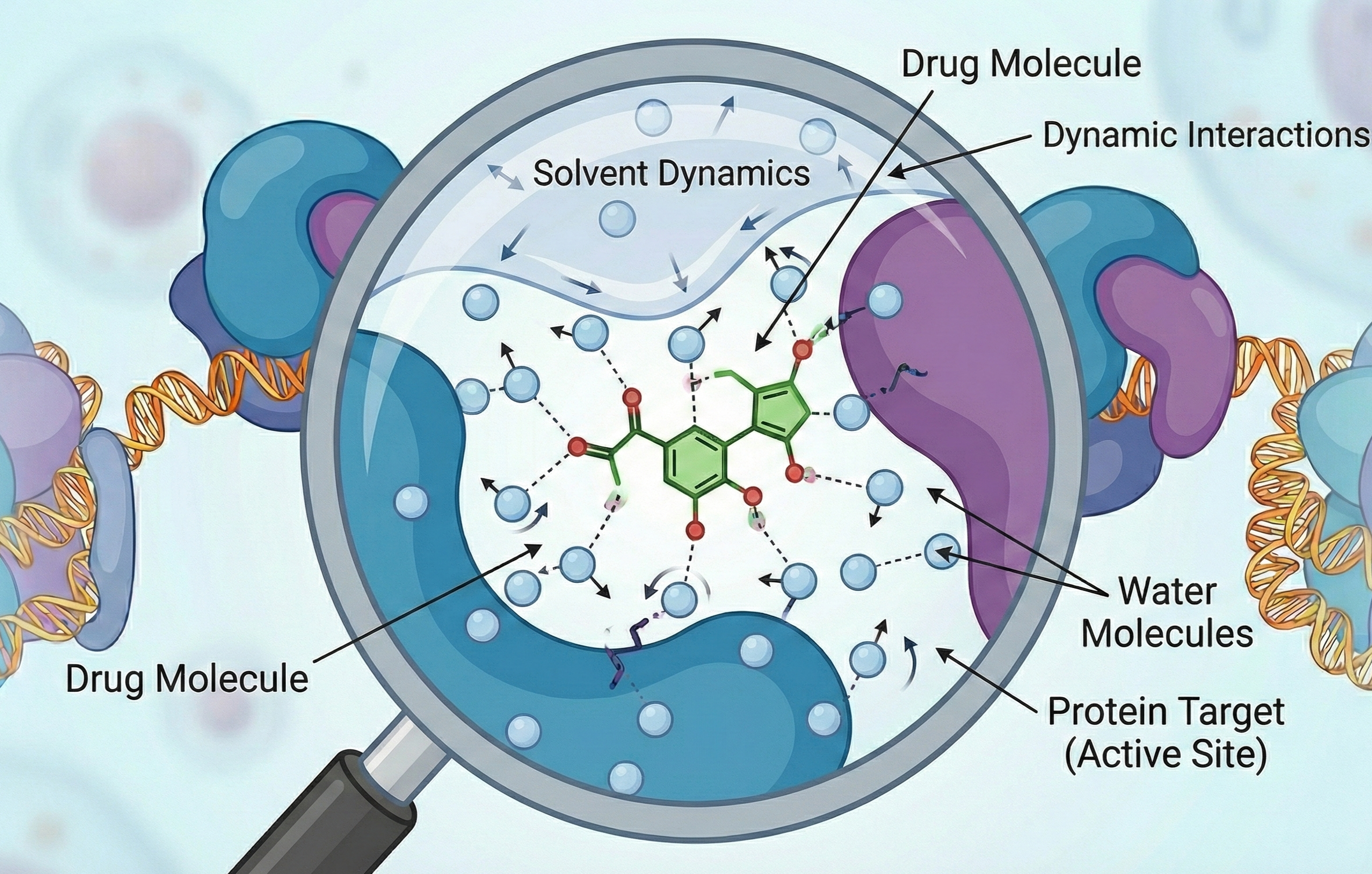
Atomic-level binding in realistic, solvent-filled environments: Water and dynamics matter for prediction!
Newly Accepted! Read our latest J. Med. Chem. Perspective (preprint), rated top 10th–25th percentile in importance.
Rethinking Drug Discovery: Dice Loading and Tossing
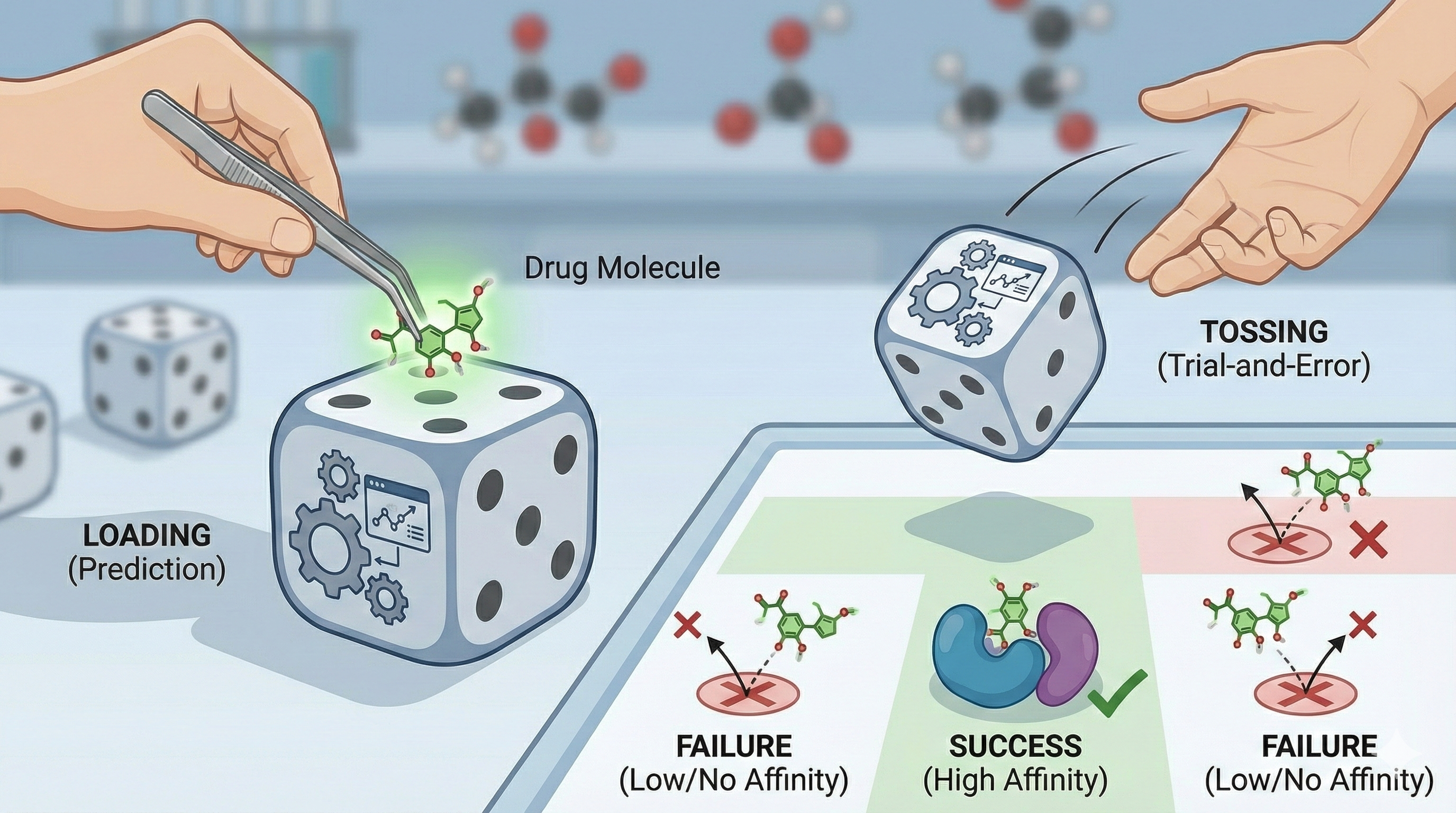
Conceptual metaphor—How well do you load the dice before you throw them? (Prediction vs. trial-and-error)
We separate drug discovery into two broad camps:
- Loading the Dice: Choosing therapeutic targets, hit, and lead molecules to predict “true-positive” clinical candidates—those actually capable of achieving a therapeutic index in humans.
- Tossing the Dice: Preclinical and clinical testing—the real-world confirmation stage, where loaded dice either land as hoped... or not. Most still fail.
| Problem | Status Quo | New Physics-Based Approach |
|---|---|---|
| Selecting Targets/Compounds | Inadequate models, incomplete/inaccurate criteria, trial-and-error | Model non-linear, multi-molecular/ionic dynamics rooted in actual cell/organismal physiology |
| Predicting Efficacy & Safety | Heavily data-driven, disconnected from native biology | Direct simulation of binding, permeability, and micro-PK under native conditions |
| Failure Rate | Remains high; little improvement in true-positive clinical prediction | Clearer rationale, new tools (e.g., WaterWorks/WATMD, Wat2Mol, CellOS) for head-to-head, first-principles comparison |
Why Does the Status Quo Fall Short?
- Myth #1: “The sum of the parts equals the whole.” Tacit linearization of cellular systems ignores complex, non-linear feedback—yielding poor human translation (just as linear models fail in planetary, atmospheric, or atomic physics).
- Myth #2: All necessary experimental variables are controlled and relevant. In reality, binding, occupancy, permeability, and in vivo distribution depend critically on unmodeled water molecules, compartmentalization, and solvation physics.
- Dogma vs. Dynamism: “Widely accepted” rules often ignore the key influence of physiological context and non-covalent interactions, leading to selection and optimization pitfalls.
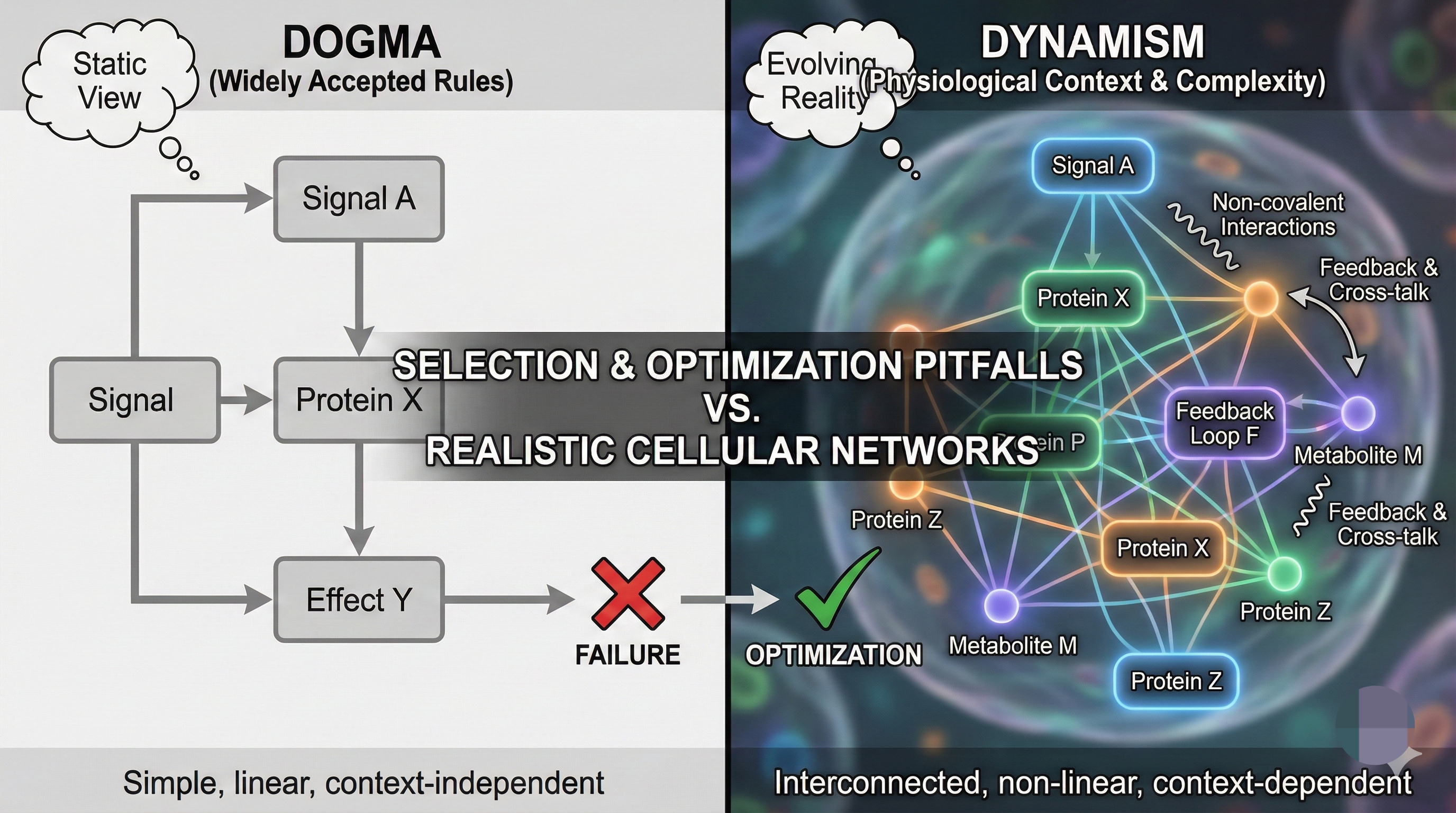
Cellular networks: Everything (really) depends on everything else! Non-linear feedback rules.
Where We’re Trailblazing:
Our in-house platforms—WaterWorks/WATMD (for solvation analysis), Wat2Mol (generative chemistry; 1st place poster, Novartis 2025), and CellOS (cellular network analysis)—address these gaps and show promise for more reliable drug-target/off-target prediction and design.
Our in-house platforms—WaterWorks/WATMD (for solvation analysis), Wat2Mol (generative chemistry; 1st place poster, Novartis 2025), and CellOS (cellular network analysis)—address these gaps and show promise for more reliable drug-target/off-target prediction and design.
The Path Forward: First Principles for Better Medicines
- Descriptive, not just predictive: New theory exposes gaps between observed, predicted, and “real-life” molecular/cellular behavior.
- Push trial-and-error downstream: Prioritize approaches that are intrinsically extrapolatable to human biology, slowing false positives and reducing attrition.
- Prepared minds over lucky guesses: Per Louis Pasteur—progress comes from foundations, not faith in serendipity.
- Intellectual honesty: To quote Neil deGrasse Tyson: “One of the greatest dilemmas in science is knowing enough to think you’re right, but not enough to know you’re wrong.” This quest is ongoing, demanding explicit tests and constant attempts at falsification.
New-generation platforms in action: Bridging chemistry, physics, computation, and systems biology for translational discovery.
There’s no silver bullet or “next big thing” on faith alone—only rigorous science and due diligence propel progress toward effective, safer medicines.
Latest Update:
Toward a First-Principles Understanding of How Cells and Small-Molecule Drugs Work under Native Physiological Conditions
Toward a First-Principles Understanding of How Cells and Small-Molecule Drugs Work under Native Physiological Conditions
Compiled August 7, 2025 · For the curious, the creative, and the critical minds of drug discovery.